About
The quantitative neuroradiology initiative (QNI) aims to deliver a means by which radiologists reporting on MR images have access
to quantitative, non-subjective data that reflects the presence or progress of a neurological condition or disease.
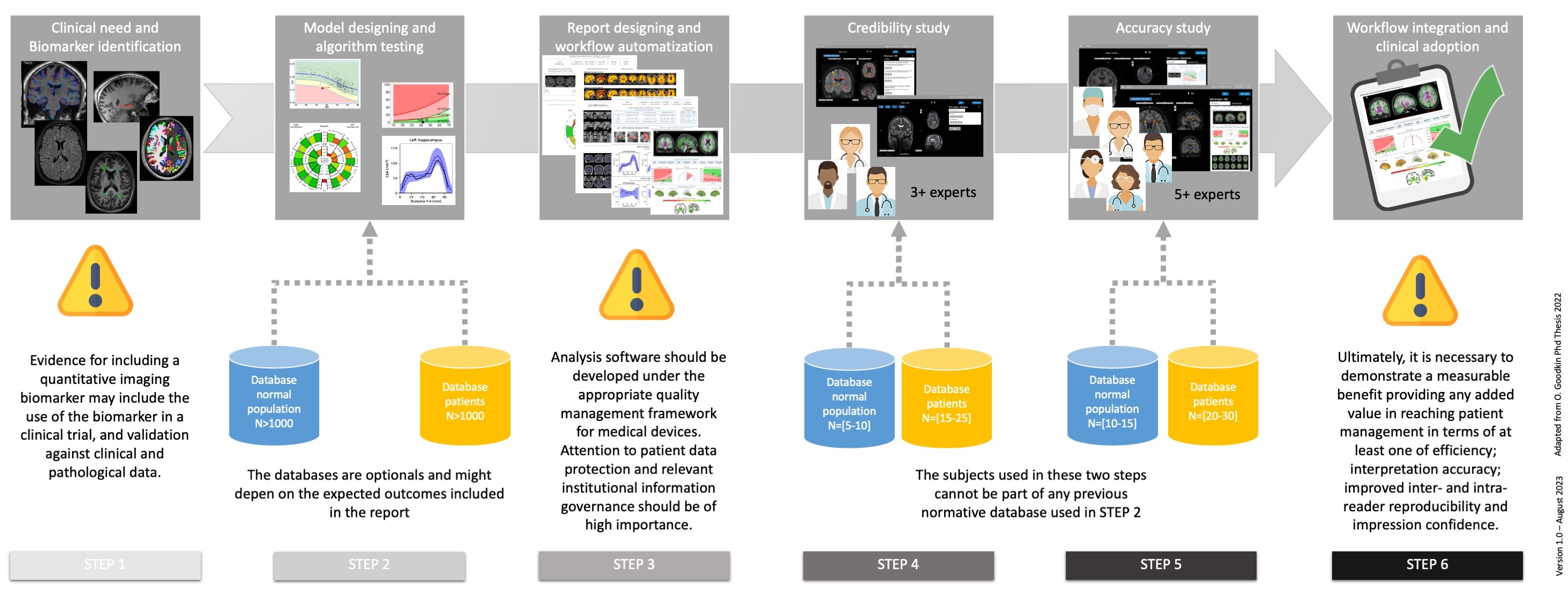
The QNI framework pathway is defined as following:
The QNI framework pathway is defined as following:
- 1 - Clinical need and Biomarker identification:
- The basis for accepting a particular imaging parameter as a correlate of the pathological process of interest should be demonstrated, and its implications for contribution to patient management should be considered. To this end, the chosen imaging biomarker should have a demonstrable physiological or pathological correlate, display sensitivity to the disease state, and be reproducible in its results.
- 2 - Model designing and algorithm testing:
- An algorithm for automated quantitative analysis of the identified biomarker(s) should be chosen or developed. This may be derived from those that have previously been used in the research setting and which may need to be adapted in some way to process clinical MRI data. At this stage disease-specific normative reference data should be identified and processed in order to provide context for comparison of an individual patient’s findings.
- 3 - Report designing and workflow automatization:
- The quantitative report should present data in a clinically, organised simply, intuitively and visually meaningful way, to ensure that the radiologist is provided with clear and accessible information that can be easily assimilated into their analysis, and their final report. The availability of quantitative information may facilitate increased adoption of standardised or structured reporting.
- 4 - Credibility study:
- Validation of the proof-of-concept quantitative analysis pipeline and biological validation with real-world data should be conducted. A credibility study should take the form of a pilot study. This should include technical validation, checks on image acquisition, postprocessing, analysis, and report generation. A limited clinical validation should be performed by experienced expert radiologists.
- 5 - Accuracy study:
- This evaluation should reproduce the radiologist’s normal reporting environment as closely as possible, with the quantitative report displayed alongside all relevant imaging series. Accuracy study assessment goals should include measurement of radiologists’ accuracy and may also include measurement of their subjective confidence and/or their reporting efficiency.
- 6 - Workflow integration and clinical adoption:
- Integration with the established clinical workflow is essential and will increase the uptake and acceptance of the reporting tool by radiologists and referring clinicians. Once the automated analysis pipeline has been embedded into the clinical workflow, in-use evaluation should be undertaken with respect to the key areas of patient management and socio-economic impact.